 |
Adeno-Associated Viruses Production by Vector Core @ GIS |
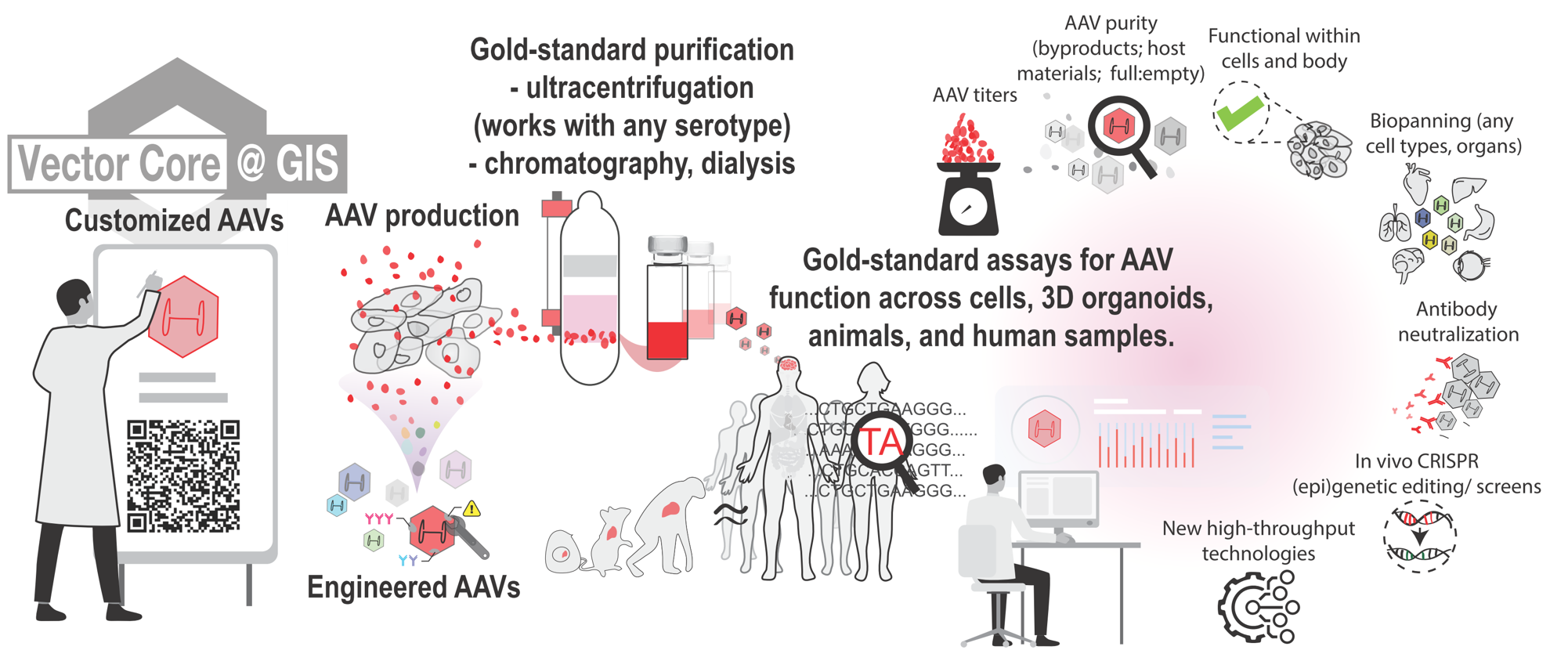
Medicines can only be effective when delivered to the right cells, tissues, and organs. One of the most promising and effective ways to do this is through Adeno-Associated Viruses (AAVs), the only viral vectors clinically approved for human use. AAVs are prevalent in the human population, benign, and highly robust for transducing cells in the body, making them the therapeutic modality of choice. The Vector Core @ GIS develops and produces high-grade AAVs for genetic and epigenetic treatments. An academic spin-off of the Molecular Therapeutics Programme, which is constituted by a team from MEL, GIS, NUS, BTI, and SERI, the Vector Core is established as a partnership with the A*STAR Research Support Centre (RSC) to generate high-grade delivery vectors. Find out how we can work together to produce customized and quality-controlled AAV vectors, identify the most efficient AAV serotypes for your cell types via biopanning, deliver genetic cargos that include CRISPR-Cas DNA and RNA -editing systems, and accelerate the translation of gene therapies into clinically relevant disease models. |
 |
Key Activities |
| The Vector Core produces high grade viral and non-viral delivery vectors including: |
1. Adeno-associated virusesThe Vector Core provides the entire production pipeline for high grade adeno-associated viruses (AAVs). AAVs are currently the only viral vectors approved for clinical gene therapy in vivo, owning to their low immunogenicity and pathogenicity. In addition, AAVs exist in many naturally occurring and engineered serotypes, each with different tropism to preferentially target cells, tissues, and organs of interest. The Vector Core can pseudotype AAVs according to research needs, including that of serotypes 1 to 9, rh10, DJ, PHP.eB, Anc80, and others. Viral production is done through triple transfection of HEK293 cells. Purification of AAVs is performed via ultracentrifugation in iodixanol density gradients, so as to isolate functional packaged AAVs containing the genetic payload from the undesired empty viral particles. Viral preps are then dialysed before Quality Control (QC) with qPCR/ddPCR titration of genomic copies, and optional services include protein assays for viral purity and functional assays for infectious units. The vector core has panels of ≥32 reporter AAVs available to test transduction efficiencies in users’ cell type of interest, and also generates AAVs customized with the payload of interest for in vitro and in vivo use. |
2. Alternative delivery methodsThe Vector Core also provides alternative delivery methods following consultation.
|
3. In vivo CRISPR-Cas deliveryThe Vector Core has significant expertise in the delivery of CRISPR-Cas systems into postnatal animals. Due to the sophisticated nature of in vivo genome-editing, consultation is necessary. |
4. ConsultationThe Vector Core can work with researchers to develop strategies for more demanding delivery applications. |
Key Services |
|
 |
Click here to find out more on Vector Core Click here for further enquiries: enquiries@rsc.a-star.edu.sg |

