 |

Contract Development & Manufacturing Organisation (CDMO) |
Restalyst has been in the research & development (R&D) of novel cancer biomarkers for in vitro diagnostic (IVD) manufacturing industry for over 15 years and is specialised in immunoassay, particularly for early detection and diagnosis of oncology and infectious diseases. Restalyst is ISO 13485 certified and is strategically located in Singapore, the heart of Southeast Asia, where it provides easy access to quality resources as well as excellent global connectivity to serve the fast-growing markets in the region and beyond. We portray a strong market presence in Southeast Asia, and we have partnerships with reputable medical laboratories, research institutions, and distributors in the region. Restalyst is capable to supply raw materials and manufacture your customised product(s) beginning from product concept to realisation. Our capability and expertise have positioned us in the market within the diagnostics and biotech industry. Restalyst offers IVD manufacturers, research institutions, and contract research organisations (CROs) a comprehensive range of products and services from “raw material to finished product”. A summary process of CDMO in Restalyst: 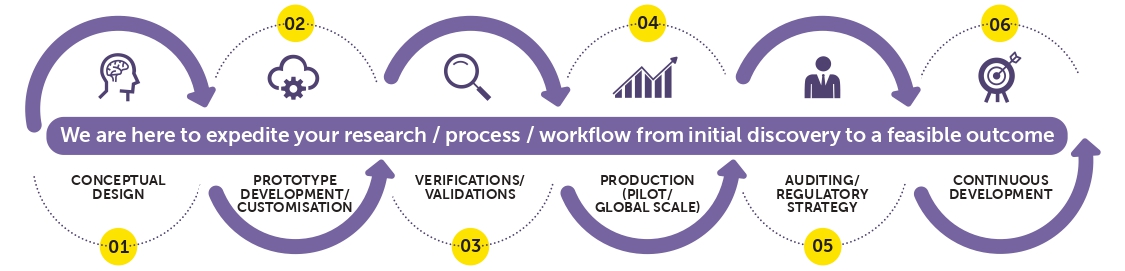 |
| Conceptual Design |
As your agile CDMO development partner, we adapt to your needs and work collaboratively to enable seamless integration of your project requirements and workflow that meets potential market demands and ultimately benefit the end-users. With the information gathered and specified by stakeholders, we will further assess its feasibility, classify IVD class, and perform preliminary risk assessment. |
| Prototype Development |
Prototypes are physical assemblies or physical products originated from conceptual mockups that will keep improving and eventually coming closer to a final engineered design with meticulous iteration. As an experienced manufacturer in IVD and life science industry, we can build a prototype and assays in a controlled lab setting equipped with cutting-edge technology. This can help us understand its potential harms to humans and uncover potential problems that will be useful for improvement and product augmentation. |
| Design Verification & Design Validation |
At this stage, design and development verification shall be performed to confirm that the design and development outputs have met the specified design and development input requirements. In addition, design and development validation shall be performed to ensure that the resulting product can meet the requirements for the specified application or intended use. |
| Pilot/Global Production |
Pilot production is defined as production of dozens to thousands of units, and the materials and processes are close or resemble those to be used in actual production. Our manufacturing facility is ISO 13485 certified for ensuring these IVDs manufactured are meeting clinical performance standards. |
| Auditing/ Regulatory Strategy |
Restalyst has an ingrained manufacturing process and international experience in the supply of IVD, notably to ASEAN countries and European markets. Hence, we are committed to provide clients our regulatory expertise ranging from initial concept discovery to regulatory approach. We have the capability to support your project(s)/ IVD(s) from regulatory perspective and prepare required documents in compliance with the up-to-date international standards for your respective country. We have also adopted and established the ASEAN Common Submission Dossier Template (CSDT) for regulatory submission to different regulatory authorities. Furthermore, we have strong credentials to assist you navigate through CE Marking process and advise on regulatory compliance for In Vitro Diagnostic Medical Devices Regulation (IVDR). |
| Continuous Development |
Our journey does not stop here, we provide solutions for today and tomorrow. We aim to continuously develop and enhance existing products which will value-add and expand your product portfolios, especially to improve clinical management of patients. We develop long-term partnerships based on the mutual interests and beliefs to bring a betterment to the world’s ever-changing clinical needs. |

To learn more about our CDMO services, contact us at info@restalyst.com or enquiries@rsc.a-star.edu.sg.

